Day 2 :
Keynote Forum
Stef Stienstra
Scientific Advisor Royal Dutch Navy, Netherlands
Keynote: The threat mitigation of emerging infectious diseases globally
Time : 08:30-09:10

Biography:
Stef Stienstra is a Strategic and Creative Development Manager in Biomedical Science, who works internationally for several medical and biotech companies as Scientific Advisory Board Member. He is also an active Reserve-Officer of the Royal Dutch Navy in his rank as Commander (OF4). For the Dutch Armed Forces, he is CBRNe specialist with focus on (micro)biological and chemical threats. He is also Manager of the group of medical- and environmental functional specialists within the 1st CMI (Civil Military Interaction) Battalion of the Dutch Armed Forces. In his civilian position, he is at this moment developing with MT-Derm in Berlin (Germany), a novel interdermal vaccination technology as well as a new therapy for cutaneous leishmaniasis for which he has won a Canadian ‘Grand Challenge’ grant. With Hemanua in Dublin (Ireland), he has developed an innovative blood separation unit, which is also suitable to produce convalescent plasma for Ebola Virus Disease therapy. He has finished both his studies in Medicine and in Biochemistry in The Netherlands with a Doctorate and has extensive practical experience in cell biology, immuno-haematology, infectous diseaases, biodefense and transfusion medicine. His natural business acumen and negotiation competence helps to initiate new successful businesses, often generated from unexpected combinations of technologies. He consults at top level management, in which his good understanding of abstract science combined with excellent skills in communication of scientific matters to non-specialists helps to get things done.
Abstract:
As curative medicine gets, compared to public health systems, generally more attention and financial support, particularly the underdeveloped countries are not well enough prepared for outbreaks of infectious diseases. In the past several Western public health institutes, like the French ‘Institut Pasteur’, the Dutch ‘Tropeninstituut‘, and many others, were prominent surveyors of contagious diseases and very active in the international mitigation of infectious diseases. In the last decennia, the investments in worldwide public health unfortunately have been reduced compared to curative healthcare. With the recent Ebola Virus Disease outbreak in West Africa, we see a new wave of growing interest to invest in Worldwide Public Health to prevent spreading of highly contagious diseases. Most public health systems in developing countries do not have proper diagnostic laboratories, quarantine procedures and treatment facilities. Non-Governmental Organisations (NGOs) helping to fight outbreaks are often better trained in curative treatments and have less skills with biological (bioweapon) threats in which military have more experience. I acclaim Bill Gates’ announcement in the New England Journal of Medicine (Bill Gates, NEJM, March 19, 2015) that all countries should identify trained military resources that would be available for outbreaks and work together to fight epidemics. New diagnostic technologies will help us worldwide in the defence against emerging contagious diseases. Especially in PCR-based systems, which are nowadays quite ruggedized, are very promising in the identification of potential outbreaks of infectious diseases in wildlife, cattle and/or the human population in developing countries.
Keynote Forum
Petra Dersch
Helmholtz Centre for Infection Research, Germany
Keynote: Global reprogramming of the Yersinia pseudotuberculosis transcriptional landscape in response to host signals
Time : 09:10-09:50

Biography:
Petra Dersch graduated in Microbiology at the University of Konstanz and at the Max-Planck-Institute for Terrestrial Microbiology Marburg. She worked as a Postdoc at the Tufts Medical School, Boston/USA, started her own group at the Freie Universität Berlin, and was Junior Research Group Leader at the Robert Koch Institute Berlin. In 2005, she was appointed at the Technische Universität Braunschweig as Associate Professor in Microbiology, and since 2008, she is Head of the Department of Molecular Infection Biology at the Helmholtz Centre for Infection Research in Braunschweig. She is member of various boards, and a current member of the study section, “Microbiology, Virology and Immunology” of the DFG. Since 2016, she is one of the Vice Presidents of the German Society for Hygiene and Microbiology. Her main research field is Molecular Pathogenesis of Enteric Pathogens. She published more than 90 original papers in peer-reviewed international journals, reviews and book chapters.
Abstract:
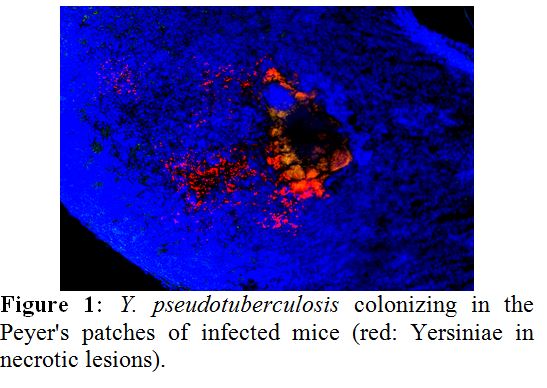
Yersinia pseudotuberculosis evolved numerous strategies to survive in environmental reservoirs and mammalian hosts. A hallmark is the ability to rapidly adjust the lifestyle upon host entry to prevent attacks by the host immune systems. The pathogen employs a plethora of control elements to fine-tune regulatory networks. To capture the range, magnitude and complexity of the underlying control mechanisms, we used comparative RNA-seq-based transcriptomic profiling under infection-relevant conditions in vitro and during the infection process in mice. We identified riboswitch-like RNA elements, a set of antisense RNAs, and previously unrecognized trans-acting RNAs, which are differentially regulated under infection conditions. We revealed a temperature- and host-induced reprogramming of important metabolic pathways, virulence traits, and discovered CRP as master regulator of non-coding RNAs. Individual regulatory RNAs, which are differentially regulated during infection, were characterized and their role in infection was elucidated using mouse infection models. Among the regulatory RNAs, which are most important for Yersinia virulence, are the Crp-dependent Csr-type regulatory RNAs found to control multiple virulence-relevant metabolic processes. Our finding highlights a novel level of complexity in which the concerted action of transcriptional regulators and non-coding RNAs adjusts the control of Yersinia fitness and virulence to the requirements of their virulent lifestyle.
Recent Publications
- Nuss AM, Beckstette M, Pimenova M, Schmühl C, Opitz W, Pisano F, Heroven AK, Dersch P (2017) Tissue dual RNA-seq allows fast discovery of infection-specific functions and riboregulators shaping host-pathogen transcriptomes. Proc Natl Acad Sci USA. 114(5):E791-E800.
- Nuss AM, Schuster F, Roselius L, Klein J, Bücker R, Herbst K, Heroven AK, Pisano F, Wittmann C, Münch R, Müller J, Jahn D, Dersch P (2016) A precise temperature-responsive Bistable Switch controlling Yersinia virulence. PLoS Pathog. 2016; 12(8):e 1006091.
- Wang H, Avican K, Fahlgren A, Erttmann SF, Nuss AM, Dersch P, Fallman M, Edgren T, Wolf-Watz H (2016) Increased plasmid copy number is essential for Yersinia T3SS function and virulence. Science. 353(6298):492-5.
- Righetti F, Nuss AM, Twittenhoff C, Beele S, Urban K, Will S, Bernhart SH, Stadler PF, Dersch P, Narberhaus F (2016) Temperature-responsive in vitro RNA structurome of Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 113(26):7237-42.
- Nuss AM, Heroven AK, Waldmann B, Reinkensmeier J, Jarek M, Beckstette M, Dersch P (2015) Transcriptomic profiling of Yersinia pseudotuberculosis reveals reprogramming of the Crp regulon by temperature and uncovers Crp as a master regulator of small RNAs. Plos Genet. 2015; 11(3):e1005087.
Keynote Forum
Sarah Louise Cosby
Queen’s University Belfast & Agri-Food & Biosciences Institute, UK
Keynote: Paramyxovirus receptor interactions: Importance in understanding cross species infection, vaccine design and disease treatment
Time : 09:50-10:35

Biography:
S Louise Cosby was appointed as the Head of Virology Branch at the Agri-Food and Biosciences Institute, UK, in 2015. She was the Chair of Microbiology in Queen’s University Belfast from 2002 and remains an Emeritus Professor. She is a Fellow of Royal College of Pathologists (London) and Fellow of the Royal Society of Biology, UK. She has served/currently serves on grant/editorial boards: BBSRC, UK; Chair/Member, Science Foundation Ireland; Deputy Chair Professional Development Committee, Microbiology Society, UK; Associate Editor, Journal of Neurovirology, USA; Review Editor, Frontiers in Microbiology; External Assessor for Appointments and Promotions in Medical Microbiology, University of Malaysia. Her research interests are: virus pathogenesis including, virus-receptor interactions, virus-induced immunosuppression and vaccine development. Her work has focused on paramyxoviruses of both human and veterinary interest, with publications/grant funding in this area.
Abstract:
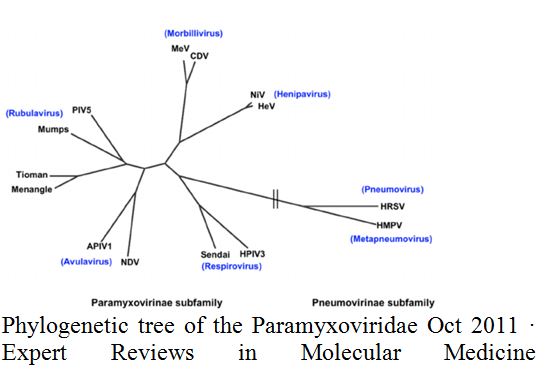
Viruses make use of cell receptors to both enter the cells and regulate cellular processes. We examined 2 important aspects of virus receptor interactions in members of the Paramyxoviridae, measles virus (MV), veterinary viruses (also in morbillivirus genus) and respiratory syncytial virus (RSV), a major cause of bronchiolitis in infants. MV can cause severe complications such as giant cell pneumonia and acute post measles encephalitis. More rarely fatal infections of the CNS, subacute sclerosing panencephalitis and in immunosuppressed individuals, measles inclusion body encephalitis occur. The World Health Organization (WHO) has set goals towards the complete eradication of MV in at least five WHO regions by 2020. We determined that both MV and RSV infection up-regulates TRPV1, TRPA1 and ASICS 3, airway receptors implicated in cough hypersensitivity and broncho-constriction in asthma and chronic obstructive pulmonary disease. We and others have also shown that veterinary morbilliviruses share common cell entry receptors with MV raising the risk of zoonotic infection. MV is thought to have evolved from the now eradicated cattle morbillivirus, rinderpest, by entering the human population during cattle domestication. This highlights the potential consequences of complete withdrawal of MV vaccination after eradication. MV vaccine is live attenuated and has very low risk of reversion, but is unlikely to be acceptable in a MV free world and may only give partial protection against morbillivirus zoonotic infection. Formalin fixed MV and RSV vaccines were used for a period in the 1960’s, but induced an altered immune response and death of some children following later infection. Based on our understanding of cross species infection new vaccines will be required. In conclusion, receptors are important players in cross-species infection as well as drug targets. Approaches to inhibit airway receptors during virus exacerbations, cell entry receptors for MV and veterinary morbilliviruses and vaccine approaches will be discussed.
Recent Publications
- Omar S, Clarke R, Abdullah Brady CH, Corry J, Winter H, Touzelet O, Power UF, Lundy F, McGarvey LPA, Cosby SL (2017) Respiratory Virus Infection Up-Regulates TRPV1 and ASICS3 Receptors on Epithelial and Neuronal Cells. Plos One DOI:10.1371/journal.pone.0171681.
- Melia M, Earle JAP EARLE, Abdullah H, Reaney, K, Willet B, Tangy F, Cosby, SL (2014). Use of SLAM, PVRL4 and HB-EGF as Cell Entry Receptors by Phocine Distemper Virus. Plos One 9(8):e106281.
- Abdullah H, Heaney LG, Cosby SL, McGarvey LPA (2014) Rhinovirus up-regulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus induced cough reflex sensitivity. Thorax 69:46-54.
- Abdullah H, Brankin B, Brady C, Cosby SL (2013) Wild Type Measles Virus Infection Up-Regulates PVRL4 and Causes Apoptosis in Brain Endothelial Cells by Induction of TRAIL. Journal of Neuropathology and Experimental Neurology 72: 681-696.
- Cosby SL (2012) Morbillivirus Cross Species infection: Are humans at risk? Future Virology 7(11):1103-1113.
Keynote Forum
Pietro Mastroeni
University of Cambridge United Kingdom
Keynote: Improved antibiotic treatments and vaccines against bacterial diseases: why do we need to understand how the pathogen interacts with the host?
Time : 11:00-11:40

Biography:
Pietro Mastroeni received a Degree in Medicine and Surgery from the University of Messina, Italy. He moved to the University of Cambridge, UK where he completed his PhD before working at the University of Newcastle, UK and then became a Research Fellow at Imperial College, University of London UK. He is currently a Reader in Infection and Immunity at the University of Cambridge, UK. He has published more than 100 papers in reputed journals, many prestigious review articles, edited two books, and serves as an Editorial Board Member for several international journals.
Abstract:
Bacterial diseases cause approximately six million deaths per year. Antimicrobial resistance is increasing and better vaccines are needed. The prevention and treatment of infections must be underpinned by an in depth knowledge of the biology and pathogenesis of the microbes and their interaction with the immune system. Empirical approaches achieve only partial success and do not allow accurate targeting of medical interventions. The location, growth status, between organs spread and interaction with cells of the immune system are key variables of the infection process that affect the efficacy of vaccine-induced immune responses and antibiotics. Our recent work has been focused on the fundamental bases of the biology of invasive Salmonella infections in the light of immune-deficiencies that predispose humans and other animals to these diseases. We have shown that Salmonella has a pathogenesis that is both intracellular and extracellular, with systemic spread in multiple body tissues and several sophisticated mechanisms that allow the bacteria to evade killing by phagocytes and disseminate in the tissues. Salmonellae are vulnerable to antibodies and complement that lyse the bacteria and/or target them to phagocytes, increasing the antimicrobial functions of host cells. We have identified phagocyte receptors, intracellular killing mechanisms and bacterial evasion strategies that affect phagocyte- and antibody-mediated killing of Salmonella. We have also determined the interactions between pathogen location, growth, spread and the efficacy of antibiotic therapy. This work lays a foundation for the development of better vaccines and antibiotic treatments for Salmonella infections and establishes principles applicable to other systemic bacterial diseases.
Recent Publications
- V Radjabova, Pietro Mastroeni, K Skjodt, P Zaccone, B de Bono, J Goodall, E Chilvers, J Juss, D Jones, J Trowsdale, A Barrow (2015) TARM1 is a novel leukocyte receptor complex-encoded ITAM receptor that costimulates proinflammatory cytokine secretion by macrophages and neutrophils. J. Immunol. 195:3149-3159.
- Pietro Mastroeni, O Rossi (2016) Immunology, epidemiology and mathematical modelling towards a better understanding of invasive non-typhoidal Salmonella disease and rational vaccination approaches. Expert Reviews of Vaccines, DOI:10.1080/14760584.2016.1189330.
- Y S Goh, K Armour, M Clark, A Grant, Pietro Mastroeni (2016) IgG subclasses targeting the flagella of Salmonella enterica serovar Typhimurium can mediate phagocytosis and bacterial killing. Journal of Vaccines and Vaccination. 7: 322.
- A Grant, O Oshota, R Chaudhuri, M Mayho, S Peters, S Clare, D Maskell, Pietro Mastroeni (2016) Genes required for the fitness of Salmonella enterica serovar Typhimurium during infection of immunodeficient gp91-/-phox mice". Infect. Immun. 84: 989-997.
- O Rossi, A Grant, Pietro Mastroeni (2017) Effect of in vivo neutralisation of tumor necrosis factor alpha on the efficacy of antibiotic treatment in systemic Salmonella enterica infections. FEMS Microbiology. doi: 10.1093/femspd/ftx002.
Keynote Forum
Alastair Fleming
Trinity College Dublin, Ireland
Keynote: Investigating the role of histone acetylation in the regulation of flocculation in Saccharomyces cerevisiae
Time : 11:40-12:20

Biography:
Alastair Fleming is an Assistant Professor in the School of Microbiology and Genetics at Trinity College Dublin, Ireland, where he is the PI of the Yeast Chromatin Group. His research group focuses on the role of ‘chromatin remodelling’ during various biological processes in yeast. Current research areas include the investigation of: (i) the epigenetic signature associated with cellular aging, (ii) the epigenetic regulation of transcription initiation, (iii) epigenetic memory during transcription elongation, and (iv), the chromatin-mediated regulation of flocculation within yeast cell populations.
Abstract:
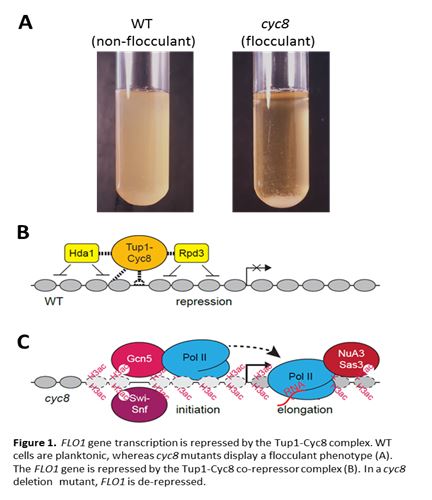
Flocculation is a stress response whereby yeast cells adhere to each other to form an aggregation which offers protection to those cells within the ‘floc’ against the outside environment. The flocculation phenotype is important in biofilm formation and in industries such as brewing. Flocculation is mediated by the expression of cell wall proteins known as flocculins. These are lectin-like proteins which bind to the mannose residues in the cell walls of neighbouring yeast cells. The dominant flocculin gene in yeast is FLO1, which is regulated by the Tup1-Cyc8 co-repressor and the Swi-Snf co-activator. Although the mechanism of FLO1 repression has been well characterised, the mechanism of FLO1 de-repression is poorly understood. We show FLO1 de-repression in a cyc8 deletion strain is accompanied by Sas3 and Ada2-dependent histone H3 lysine-14 acetylation at the FLO1 promoter and ORF, together with Swi-Snf recruitment and histone eviction at the promoter. In the absence of Ada2 and Sas3-dependent H3 lysine-14 acetylation, Swi-Snf recruitment and histone eviction proceed at the de-repressed FLO1 promoter, but FLO1 transcription is reduced. Following the conditional depletion of Cyc8 via anchor-away, we show RNA polymerase II (RNAP II) is recruited to the de-repressed FLO1 promoter in a bi-phasic manner concomitant with a similar pattern of histone acetylation. In the absence of Sas3 and Ada2-dependent H3 acetylation, histone eviction and RNAP II recruitment at the FLO1 promoter still occur, however RNAP II is absent from the gene coding region. This suggests that in the absence of Cyc8, Sas3 and Ada2-dependent histone H3K14 acetylation is not required for histone eviction and RNAP II recruitment at the FLO1 promoter, but is required to enable transcription elongation to occur.
Recent Publications
- Young CP, Hillyer C, Hokamp K, Fitzpatrick DJ, Konstantinov NK, Welty JS, Ness SA, Werner-Washburne M, Fleming AB, Osley MA (2017) Distinct histone methylation and transcription profiles are established during the development of cellular quiescence in yeast. BMC Genomics. 18(1):107.
- Church M, Smith KC, Alhussain MM, Pennings S, Fleming AB (2017) Sas3 and Ada2(Gcn5)-dependent histone H3 acetylation is required for transcription elongation at the de-repressed FLO1 gene. Nucleic Acids Res.
- Haran J, Boyle H, Hokamp K, Yeomans T, Liu Z, Church M, Fleming AB et al. (2014) Telomeric ORFs (TLOs) in Candida spp. encode mediator subunits that regulate distinct virulence traits. PLoS Genet. 10(10).
- Fleming AB, et al. (2014) The yeast Cyc8-Tup1 complex cooperates with Hda1p and Rpd3p histone deacetylases to robustly repress transcription of the subtelomeric FLO1 gene. BBA-GRM. 1839(11):1242-55.
- Barski A, Chepelev I, Liko D, Cuddapah S, Fleming AB, et al. (2011) Pol II and its associated epigenetic marks are present at Pol III-transcribed noncoding RNA genes. Nat Struct Mol Biol. 17: 629-634
- Bacteriology, Virology, Veterinary Microbiology, Mycology, Phycology, Environmental and Agricultural Microbiology
Location: Redwood Suite C

Chair
Joachim Wink
Helmholtz Centre for Infection Research, Germany

Co-Chair
Stef Stienstra
Scientific Advisor Royal Dutch Navy, Netherlands
Session Introduction
Maria Angela Cunha
University of Aveiro, Portugal
Title: Photodynamic inactivation of phytopathogenic fungi with natural and synthetic photosensitizers
Time : 11:40-12:05

Biography:
Angela Cunha is an Assistant Professor in the Biology department of the University of Aveiro, Portugal and a Researcher at the Centre for Environmental and Marine Studies. She has been involved in lecturing graduate and post graduate courses in the fields of Microbiology and Genetics and is currently the Director of the Bachelor Course in Biology. Her main research field is the distribution and activity of microorganisms in the environment, microbial bioremediation tools and interactions of microorganisms with plants in the perspectives of disease control, plant growth promotion and microbe-assisted phytoremediation.
Abstract:
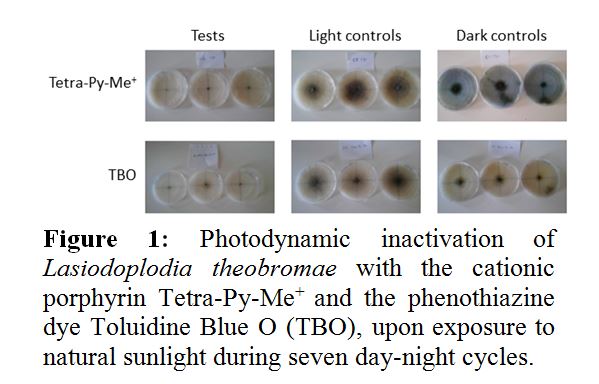
Diseases caused by phytopathogenic fungi on vine and fruit trees are associated with important economic losses. With the aim of reducing the environmental impacts of traditional fungicides used in agriculture, innovative approaches to inactivate fungi in environmental matrices are being investigated. In this context, the photodynamic effect, in which the combination of a photosensitizer, visible light and molecular oxygen leads to the formation of cytotoxic species of oxygen, represents a promising perspective. The objective of this work was to evaluate the efficiency of photodynamic inactivation (PDI) of model phytopathogenic fungi (Lasiodoplodia theobromae and Botrytis cinerea) with natural (curcumin and riboflavin) and synthetic (cationic porphyrin Tetra-Py-Me+ and toluidine blue O) photosensitizers. Exposure to natural sunlight during 7 day-night cycles, in presence of 500 µM of Tetra-Py-Me+ or 500 µM toluidine blue O, attenuated the growth of L. theobromae by 35% and 26%, respectively (Figure 1). Curcumin and riboflavin failed to cause significant inactivation. Light alone (light control) and exposure to the photosensitizer in the absence of light (dark control) did not affect the growth of L. theobromae. On the contrary, the growth of B. cinerea was significantly inhibited by light alone. Tetra-Py-Me+ and toluidine blue O caused 90% and 93% attenuation of growth, respectively, in relation to the dark control. PDI with the natural photosensitizers was also not significant. The results confirm that PDI with porphyrins or phenothiazines may be further explored as a viable alternative to chemical fungicides in the control of phytopathogenic fungi. However, repeated treatments may be required considering that in field conditions, fungi may recover from sub-lethal damage, during the night period.
Recent Publications
- Fernández L, Esteves VI, Cunha A, Schneider RJ, Tomé JPC (2016) Photodegradation of organic pollutants in water by immobilized porphyrins and phthalocyanines. Journal of Porphyrins and Phthalocyanines 20:150–166.
- Rocha DM, Venkatramaiah N, Gomes MC, Almeida A, Faustino, MA, Paz FA, Cunha Â, Tomé JP (2015) Photodynamic inactivation of Escherichia coli with cationic ammonium Zn (ii) phthalocyanines. Photochemical & Photobiological Sciences 14(10):1872-1879.
- Beirão S, Fernandes S, Coelho J, Faustino MAF, Tomé JPC, Neves MGPMS, Tomé A, Almeida A, Cunha A (2014) Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin. Photochemistry & Photobiology 90(6):1387–1396.
- Gomes MC, Silva S, Faustino MAF, Neves MGPMS, Almeida A, Cavaleiro, JAS, Tomé JPC, Cunha A (2013) Cationic galactoporphyrin photosensitisers against UV-B resistant bacteria: oxidation of lipids and proteins by 1O2. Photochemical & Photobiological Sciences 12:262–271.
- Gomes MC, Woranovicz-Barreira SM, Faustino MAF, Fernandes R, Neves MGPMS, Tomé AC, Gomes NCM, Almeida A, Cavaleiro, JAS, Cunha A, Tomé JPC (2011) Photodynamic inactivation of Penicillium chrysogenum conidia by cationic porphyrins. Photochemical & Photobiological Sciences 10:1735-1743.
Rehana A.Gilani
Shaheed Zulfiqar Ali Bhutto Medical University, Pakistan
Title: Resistance profile of ESBL positive uropathogenic E. coli in a tertiary care teaching hospital in Islamabad, Pakistan

Biography:
Rehana A Gilani has completed her MPhil from a teaching hospital in Pakistan and has tried to incorporate research in the regular curriculum of the newly established university.
Abstract:
Introduction & Aim: Escherichia coli is the most common Gram negative organism causing both community, as well a,s hospital acquired Urinary Tract Infection (UTI). This study was conducted to find out the prevalence of uropathogenic E. coli and to demonstrate ESBL phenomenon and their antimicrobial susceptibility patterns. The genetic characterization of ESBL producing isolates was also done to demonstrate the genes conferring drug resistance to these organisms in our institution using conventional PCR.
Materials & Methods: This research was a prospective, non- randomized, descriptive study. A pro forma was used as a tool for data collection. The study was conducted in the Microbiology Laboratory, Department of Pathology, PIMS, Shaheed Zulfiqar Ali Bhutto Medical University for 8 months. 140 urine non-duplicate samples of patients with UTI yielding growth of E. coli were selected and their susceptibility profile was determined.
Results: Urine samples of 140 patients yielding E. coli were enrolled in the study. There were 81 (58%) females and 59 (42%) males. Patients included in the study were from 12-86 years of age. The ESBL phenomenon was confirmed by double disc method, which demonstrated that 80 (57%) samples were positive and 60 (43%) were negative.
Conclusion: Our results showed emergence of multidrug resistant ESBL producing E. coli in our set up, which is a very serious problem due to non-availability of new antibiotics and also the element of poverty, due to which the patients are unable to afford the prescribed drugs.
Herbert B. Allen
Drexel University College of Medicine, USA
Title: The Pathway to Alzheimer’s Disease

Biography:
Herbert B Allen specialties include dermatology and dermatopathology, skin pathology and fungal infections. He is a graduate of Johns Hopkins University School of Medicine. He has served on the boards of the American Society of Dermatology and the American College of Physicians and has published over 30 scientific articles in the fields of dermatology and dermatopathology. He is the author of ‘Keywords’ in Dermatology, a book on the language of dermatology. He's board-certified with the American Board of Dermatology and the American Board of Pathology. He is currently an Emeritus Professor in the department of Dermatology where he served as chair of the department for 14 years.
Abstract:
The pathway to Alzheimer’s disease (AD) follows a route which many chronic diseases utilize: microbes make biofilms that activate the innate immune system which ultimately leads to tissue destruction. The primary microbes involved are very likely dental and Lyme spirochetes. Both have been found by PCR; additionally, Lyme spirochetes have been cultured from affected brains. Both are known to make biofilms, and these biofilms have receptor sites for Toll-like receptor 2 (TLR2). In its activity as a first responder, TLR2 makes use of the myeloid differentiation 88 pathway to kill invading microorganisms. This pathway generates NFκB and TNFα and these molecules in turn appear not only to be responsible for destruction of neurons, but also to be responsible, in large part, for the creation of beta amyloid. (Aβ) The Aβ has been previously thought to be primary in the AD pathogenesis, but it turns out to be antimicrobial. Neither the TLR2 nor the Aβ can penetrate the biofilms and thus they destroy the surrounding tissue as “innocent bystanders”. The recent findings of intracellular, in contrast to extracellular, biofilms complicate the system further. Adding to the pathway are factors that contribute to the disease process and make AD worse. Among these are medications such as haloperidol and chemicals such as nicotine and beta methyl aminoalanine which are known to be biofilm dispersers. Diabetes is known to make AD worse; the likely mechanism for this is an increase in biofilms caused by hyper osmolality in the serum. Thus, making or breaking (dispersing) biofilms eventuates in more biofilms and more disease. Only a few factors ameliorate AD: low levels of vitamins D3 and K2 upregulate TLR2 causing more disease activity. Higher serum levels do the opposite. Vitamin E and L-serine are quorum sensing inhibitors; and, when present, aid in preventing biofilm formation. Last, if the microbes are killed (by penicillin) before they reach the brain or before they make biofilms, there would likely be no disease at all.
Anna Krasowska
University of Wroclaw, Poland
Title: The capric acid from Saccharomyces boulardii as an antifungal agent: a mechanism study
Time : 14:00-14:25

Biography:
Anna Krasowska is an assistant professor at the Department of Biotransformation, University of Wroclaw, Poland. Currently they are involved in the isolation and characterization of biosurfactants produced by arctic microorganisms. She has also examined the activity of lipases and proteases released into the environment by microorganisms isolated from different environments. Her research interests lie in multidrug resistance of pathogenic microorganisms like Saccharomyces cerevisiae, yeast and Candida albicans.
Abstract:
Candida albicans is a pathogenic yeast-like fungus that causes exo- and endogenous infections. C. albicans strains exhibit multidrug-resistance to commonly used antifungal agents which correlates with overexpression of Cdr and Mdr efflux pumps located in the plasma membrane. Growing resistance of pathogenic C. albicans strains to many classes of antifungal drugs has stimulated efforts to find new agents to combat more invasive infections. A selected number of probiotic organisms, Saccharomyces boulardii among them, have also been tested as potential biotherapeutic agents. S. boulardii is a yeast strain that has been shown to have applications in the prevention and treatment of intestinal infections caused by microbial pathogens. We have similarly shown that S. boulardii secretes capric acid (C10:0), which is most effective in inhibiting essential virulence factors of C. albicans, especially morphological transition, partial adhesion, as well as biofilm formation. Our latest research on the mechanism of action of capric acid and its influence on the C. albicans’ cells clearly show its interaction with the plasma membrane. Capric acid decreases fluidity, while increasing the potential of the plasma membrane. For these reasons, we have probably not observed antifungal activity of amphotericin B in the presence of capric acid. The antagonism between capric acid and amphotericin B is a strong indication for physicians to not use both compounds simultaneously in the treatment of candidiasis.
Recent Publications
1. Sellam A, Whiteway M (2016) Recent advances on Candida albicans biology and virulence F1000Res 26:2582-2590.
2. Sanguinetti M, Posteraro B, Lass-Flörl C (2015) Antifungal drug resistance among Candida species: mechanisms and clinical impact Mycoses 58:2-13
3. Kumar S, Singhi S, Chakrabarti A, Bansal A, & Jayashree M (2013) Probiotic use and prevalence of candidemia and candiduria in a PICU Pediatric Critical Care Medicine 14: e409-e415.
4. Martin I, Tonner R, Trivedi J, Miller H, Lee R, Liang X, Rotello L, Isenbergh E, Anderson J, Perl T, & Zhang X (2017) Saccharomyces boulardii probiotic-associated fungemia: questioning the safety of this preventive probiotic's use Diagnostic Microbiology and Infectious Disease 87:286–288.
5. TomiÄić Z, Zupan J, Matos T, & Raspor P (2016) Probiotic yeast Saccharomyces boulardii (nom. nud.) modulates adhesive properties of Candida glabrata Medical Mycology 54:835-845.
Tatyana Tracevska
University of Latvia, Latvia
Title: Interdisciplinary studies on Ayurvedic herbal formulation showing antibacterial effect on methicillin resistant Staphylococcus aureus
Time : 14:25-14:50

Biography:
Tatyana Tracevska is an Associate Professor of University of Latvia, Faculty of Medicine. She has her expertise in Molecular Microbiology, focusing on drug resistance mechanisms, persistent infections and epidemiological studies of pathogens such as Mycobacterium tuberculosis, Staphylococcus aureus Chlamydia trachomatis. In 2009, she received ESCMID (European Society of Clinical Microbiology and Infectious Diseases) and bioMérieux Award for Advances in Clinical Microbiology in East Central or Central Europe, and for the project entitled, luxS gene as a novel diagnostics for invasive CoNs. From 2010 to 2012, she was involved as a Head Scientist in European Social Fund co-founded project “Capacity building for interdisciplinary biosafety research” by University of Latvia. From 2016, she is a Head of effective collaboration project between University of Latvia and Arya Vaidya Pharmacy Baltics “Development of a novel herbal product for wound healing using the method of lamellar gel phase emulsion”.
Abstract:
Statement of the Problem: Emerging bacterial resistance in response to antimicrobial therapy is a world-wide problem nowadays, stimulating the search for new antimicrobial compounds. Combinatory therapy including antibacterial agents and herbal extracts, is able to combat bacterial resistance, is the promising perspective. The object of present study is a polyherbal formulation, Jathyadi Thailam, based on 13 herb infusion in coconut oil, used in Ayurvedic medicine for chronic wound (e.g. Diabetic foot ulcers) and burns healing.
Methodology & Theoretical Orientation: The antimicrobial activity of this formulation for biofilm forming and drug resistant bacteria such as methicillin resistant staphylococci, or extended spectrum beta-lactamase producing bacilli has been studied. The antibacterial efficacy of the herbal oil and its polar and nonpolar crude extracts was tested by agar dilution method and by broth microdilution method for mostly common isolates from diabetic wounds. Reference strains and clinical isolates from diabetic foot ulcers isolated in Latvian hospital were analyzed.
Findings: Interestingly, the bacteriostatic effect was shown by Jathyadi Thailam by the agar dilution method for methicillin resistant Staphylococcus aureus ATCC 38592, drug susceptible S.aureus ATCC 2848 and clinical S.aureus strain, Pseudomonas aeruginosa ATCC 2843, Enterococcus faecalis ATCC 29212 and biofilm producing S.epidermidis reference strains. No inhibition was observed for Klebsiella pnemoniae ATCC 2558, Proteus mirabilis ATCC 432351, Echerichia coli ATCC 25922 and for clinical Prowidencia spp. and ESBL producing K.pneumoniae strain. Minimal bactericidal concentration (MBC) was determined for the crude herbal polar and nonpolar extracts by the broth microdilution method. The MBC was determined as 15.6 mg/ml and 31.2 mg/ml by polar and nonpolar extracts, respectively, for MRSA ATCC 38592, 7.8 mg/ml by both extracts for S.aureus ATCC 2848, 1.95 mg/ml by both extracts for biofilm producing S.epidermidis and 62.5mg/ml by nonpolar extract for P. aeruginosa.
Conclusion & Significance: To conclude, both herbal crude extracts and the final herbal oil were found to be more effective for Gram positive bacteria, when for Gram negative. The formulation was effective against Staphyloccus aureus, the bacteria predominant in chronic diabetic wounds. Further studies will be focused on development of herbal suspension with better skin penetrating properties and aimed to inhibit the growth of Gram negative bacteria.
Recent Publications
- Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM et al. (2008) Survey of bacterial diversity in chronic wounds using Pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol 8: 43.
- Dhande Priti P, Raj S, Kureshee N et al. (2012) Burn wound healing potential of Jatyadi formulations in rats. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 3(4): 747-754.
- Boutoille D, Fe Ìraille A, Maulaz D et al (2008) Quality of life with diabetes-associated foot complications: comparison between lower-limb amputation and chronic foot ulceration. Foot and Ankle International, 29(11):1074–1078.
- Payne D, Gwynn M, Holmes DJ et al (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nature Reviews Drug Discovery 6(1):29– 40.
- Menon SS, Pednekar S and Singh A (2011) Wound healing efficacy of Jatyadi Taila: in vivo evaluation in rat using excision wound model, J. Ethnopharmacol. 138: 99-104.
Stef Stienstra
Scientific Advisor Royal Dutch Navy, Netherlands
Title: Cooperation in public health to fight infectious diseases in developing countries is good for the global economy
Time : 14:50-15:15

Biography:
Stef Stienstra works internationally for several medical and biotech companies as scientific advisory board member and is also an active reserve-officer of the Royal Dutch Navy in his rank as Commander (OF4). For the Dutch Armed Forces, he is CBRNe specialist with focus on (micro)biological and chemical threats and medical- and environmental functional specialist within the 1st CMI (Civil Military Interaction) Battalion of the Dutch Armed Forces. He is managing an EU CBRN CoE public health project in West Africa on behalf of Expertise France. In his civilian position, he is currently developing with MT-Derm in Berlin (Germany) a novel interdermal vaccination technology as well as a new therapy for cutaneous leishmaniasis for which he has won a Canadian ‘Grand Challenge’ grant. With Hemanua in Dublin (Ireland) he has developed an innovative blood separation unit, which is also suitable to produce convalescent plasma for Ebola Virus Disese therapy. He has finished both his studies in Medicine and in Biochemistry, Netherlands with a doctorate and has extensive practical experience in cell biology, immuno-haematology, infectous diseases, biodefense and transfusion medicine.
Abstract:
Public health systems are not always prepared for outbreaks of infectious diseases. Although in the past several public health institutes, like the French ‘Institut Pasteur’ and the Dutch ‘Tropeninstituut ‘, were prominent surveyors of infectious diseases, the investments in worldwide public health have decreased. Now more attention is given to curative healthcare compared to preventive healthcare. The recent Ebola Virus Disease outbreak in West Africa initiated a new wave of interest to invest in Worldwide Public Health to prevent outbreaks of highly contagious diseases. Zoonotic diseases are threatening as the population does not have natural nor artificial (from vaccination) immune response to new diseases like in the Ebola Virus Disease outbreak in 2014. The new strain of the Ebola Virus in West Africa was slightly less lethal, compared to other Ebola Virus strains, but the threat of spreading was far bigger as it had a longer incubation time. Most public health systems are not trained well enough to mitigate highly infectious and deadly disease outbreaks. NGO’s helping to fight the outbreak are often better trained in curative treatments and have less experience with biological (bioweapon) threats for which the military are trained for. The UNMEER mission was unique in this. It was a setting in which military and civilian actors cooperate in fighting a biological threat. Protection is essential for health workers. Smart systems must be developed to prevent further spreading of the disease, but it is not only the biosafety, which has to be considered, but also the biosecurity, as misuse of extremely dangerous strains of microorganisms cannot be excluded. Several zoonotic infectious diseases, like anthrax, smallpox and hemorrhagic fevers are listed as potential bioweapons. Therefor both biosafety and biosecurity have to be implemented in all measures to fight outbreaks of highly infectious diseases.
Recent publications
1. Moon S et. al. (2015) Will Ebola change the game? Ten essential reforms before the next pandemic. The report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. Lancet. 386(100069): 2204-21.
2. Kamradt-Scott A et. al. (2015) WHO must remain a strong global health leader post Ebola. Lancet 385(9963):111.
3. Kieny MP, Dovlo D (2015) Beyond Ebola: a new agenda for resilient health systems. Lancet 385(9963):91-92.
4. Cenciarelli O et. al. (2015) Viral bioterrorism: Learning the lesson of Ebola virus in West Africa 2013-2015. Virus Research 210: 318-326.
5. Abramowitz SA et. al. (2015) Social science intelligence in the global Ebola response. Lancet 385(9965): 330.

Biography:
Herbert B Allen specialties include dermatology and dermatopathology, skin pathology and fungal infections. He is a graduate of Johns Hopkins University School of Medicine. He has served on the boards of the American Society of Dermatology and the American College of Physicians and has published over 30 scientific articles in the fields of dermatology and dermatopathology. He is the author of ‘Keywords’ in Dermatology, a book on the language of dermatology. He's board-certified with the American Board of Dermatology and the American Board of Pathology. He is currently an Emeritus Professor in the department of Dermatology where he served as chair of the department for 14 years.
Abstract:
We recently have found biofilms in an intracellular position in Alzheimer’s disease (AD), molluscum contagiosum (MC), and psoriasis. We have noted them also in leprosy. With AD, the biofilms are made primarily by dental and Lyme spirochetes; with MC, it is the molluscum virus that “hijacks” the cell DNA and makes them; and with psoriasis, it is streptococci, primarily in the pharynx that make them. In leprosy, we have observed them inside the “globi” that are prominent in lepromatous leprosy, and they are made by Mycobacterium leprae. Thus, spirochetes, DNA viruses, gram positive bacteria, and mycobacteria can be added to the list of microbes that make intracellular biofilms. Intracellular biofilms add another layer of protection to the slime coating already in place. We have found these biofilms with routine histopathologic staining with PAS and Congo red (CR) stains. The PAS stains the extracellular polysaccharides (EPS) that are an essential ingredient in biofilms. The CR stains the amyloid that forms the infrastructure of biofilms and is another essential element. These intracellular biofilms have both been clearly shown in these diseases and exist alongside the extracellular biomasses that have previously been found. Limits to the evaluation of these findings involve the resolution power of light microscopy, selection of the tissue involved (in psoriasis, it is the tonsils, not the skin, where the biofilms are found), and the lack of a test, such as culture, to confirm the findings. The size of the organisms is important to make a biofilm, the microbes must have 10 organisms in any direction to begin the process. The resulting aggregate must then fit within the cell cytoplasm. The size ranges from 3 nM in MC to 25µM in Borrelia. These clearly are spatially able to fit within the cells. These findings are novel as to finding biofilms intracellularly in the skin and are the second time in history that a virus has been shown to make biofilms.
- Young Research Forum
Location: Redwood Suite C

Chair
Alastair Fleming
Trinity College Dublin, Ireland
Session Introduction
Nicolas Le Goff
Enzyme and Cellular Engineering Laboratory, UTC, France
Title: Recalcitrant polymers biodegradation by phylloplanic bacterial and fungal isolates: identification of efficient strains and characterization of the degradation products
Time : 17:15-17:30

Biography:
Nicolas Le Goff, after graduating as a Biological Engineer at the UTC, mostly specialized in healthcare and drug delivery, he wanted to diversify his skills and started his PhD project at the Enzymatic and Cellular Engineering (GEC) Laboratory and the Integrated Transformation of the Renewable Matte (TIMR) Laboratory of the UTC. He truly believes that biomimetism and nature can inspire research and will allow discovering and developing alternatives to actual polluting industrial processes. The approach of this work can be adapted to different environmental media to identify microbial strains of interest for a large scale of applications.
Abstract:
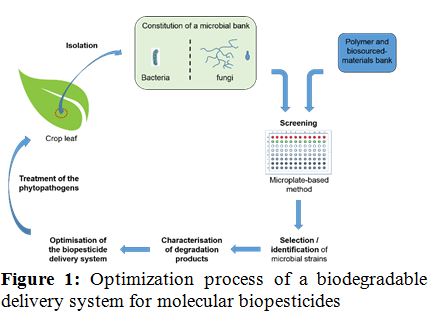
Statement of the Problem: The excessive use of chemical pesticides in agriculture represents healthcare and environmental concerns. A possible alternative to fight against phytopathogens is the use of biopesticides such as secondary metabolites or enzymes. But the stabilization of those molecules to obtain long term and higher efficiency on plant is still an issue. One proposed solution is the encapsulation of biopesticides in polymeric materials. Thus, the biodegradability of those materials by microbes present on the crops leaves has to be evaluated for the selection of the most suitable encapsulating agent. The work presented here consists in the isolation of microorganisms from crops, the screening of their degradation abilities and the identification of efficient strains.
Methodology & Theoretical Orientation: First, bacteria and fungi have been isolated from different crops leaves (corn, rapeseed, cabbage and sugar beets). Two isolation techniques were used: the suspension dilution technique and the foliar imprints method. A small scale microplate-based screening method was optimized to select both bacteria and fungi able to grow with different polymeric materials as sole carbon source. Secondly, the degradation products of the polymers were characterized with different analytical techniques (GC-MS, SEC, isotopic labeling) to identify the metabolic pathways and to prevent to impact the environment with toxic metabolites.
Findings: 117 fungal and 212 bacterial isolates have been obtained and screened for the degradation of several polymers (pHEMA, pMMA, pNIPAM, pAcrylate, pAcrylamide and bio-sourced polymer). First results show good abilities of the microbial bank to degrade mainly pHEMA and pAcrylamide.
Conclusion & Significance: Numerous microbial strains isolated in this work are able to degrade polymeric and biobased materials. This should allow the development of new biodegradable vectorization systems for the stabilization of molecular biopesticides as an alternative to chemicals, but also of bioremediation processes.
Recent Publications
- Transparency Market Research (2016) Biopesticides Market - Global Industry Analysis, Size, Share, Growth and Forecast 2015 – 2023, 95.
- Villaverde JJ (2014) Chapter 15 – Challenges of Biopesticides Under the European Regulation (EC) No. 1107/2009: An Overview of New Trends in Residue Analysis. Studies in Natural Products Chemistry 43: 437-482.
- Chilukoti N (2010) Biotechnological approaches to develop bacterial chitinases as a bioshield against fungal diseases of plants. Critical Rewiews in Biotechnology 30(3): 231-241.
- Salgado M (2015) Encapsulation of resveratrol on lecithin and b-glucans to enhance its action against Botrytis cinerea. Journal of Food Engineering 165:13–21.
- Ya-Ting X (2011) Molecularly imprinted polymer microspheres enhanced biodegradation of bisphenol A by acclimated activated sludge. Water Research 45(3): 1189-1198.
Katherine Hayes
Cork Institute of Technology, Ireland
Title: Macrolide and lincosamide resistance amongst Group B Streptococci – a growing concern?
Time : 17:30-17:45

Biography:
Kate Hayes is a Postgraduate student in the Biological Sciences at the Department of Cork Institute of Technology. Upon completion of her undergraduate degree in Biomedical Science, she was awarded a RÍSAM Scholarship by CIT. With interests in clinical microbiology and molecular biology, she is undertaking research on Group B Streptococci with a project entitled “Group B Streptococci - molecular epidemiology, pathogenic profiling and control strategies” under the joint supervision of Dr. Lesley Cotter and Dr. Fiona O’ Halloran.
Abstract:
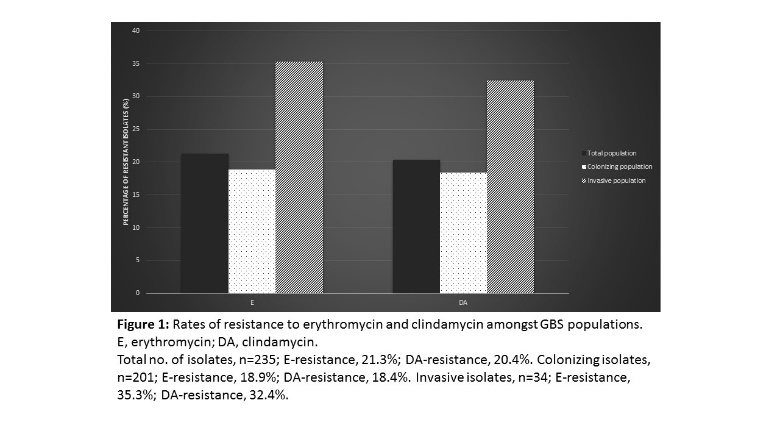
Group B Streptococcus (GBS) is the leading cause of invasive neonatal disease worldwide, with fatality rates of up to 10%. It colonizes the genitourinary tract of approximately 30% of pregnant women and in recent years it has become a growing concern amongst non-pregnant adults. Penicillin remains the first line of treatment for GBS infections, however there have been reports of reduced sensitivity in some countries. Clindamycin and erythromycin are used in cases of beta-lactam allergy, with rates of resistance to these antibiotics continuing to rise. This study aimed to investigate the incidence of antimicrobial resistance in a clinical population of GBS isolates (n=235). Antimicrobial susceptibility testing was performed according to EUCAST guidelines and the underlying mechanism of resistance was determined, both phenotypically and genotypically. Isolates were further characterized based on their serotype using molecular methods and the prevalence of the hyper-virulent ST-17 clone was investigated. Resistance to erythromycin and clindamycin was observed in 21.3% and 20.4% of the total population respectively. The c-MLSB phenotype was the most common, detected in 62% of isolates, followed by i-MLSB (20%) and M (18%) phenotypes. The rare L phenotype was also confirmed in the clinical population. ErmB was the predominant genetic determinant, identified in 84% of isolates. Both mefA/E and ermTR were each present in 18% of the isolates. Molecular serotyping analysis revealed capsular types Ia, III and II were the most common serotypes (28.1%, 24.7% and 14% respectively). Evidence of capsular switching was observed in one GBS isolate, which has implications for vaccines that target the capsular polysaccharide. This work highlights emerging trends in antimicrobial resistance amongst the Irish GBS population and emphasizes the need for continued monitoring of antibiotic resistance and serotype distribution in the population.
Recent Publications
1. Hawkins PA, et al. (2017) Cross-resistance to lincosamides, streptogramins A and pleuromutilins in Streptococcus agalactiae isolates from the USA. Journal of Antimicrobial Chemotherapy 5: 4544.
2. Arana DM, et al. (2014) First clinical isolate in Europe of clindamycin-resistant group B Streptococcus mediated by the lnu(B) gene. Revista española de quimioterapia: publicacioÌn oficial de la Sociedad Española de Quimioterapia 27: 106–9.
3. Lamagni TL, et al. (2013) Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991-2010. Clinical infectious diseases 57: 682–688.
4. Malbruny B, et al. (2011) Cross-resistance to lincosamides, streptogramins A, and pleuromutilins due to the lsa(C) gene in Streptococcus agalactiae UCN70. Antimicrobial Agents and Chemotherapy 55: 1470–4.
5. Kimura K, et al. (2008) First Molecular Characterization of Group B Streptococci with Reduced Penicillin Susceptibility. Antimicrobial Agents and Chemotherapy 52: 2890–2897.
Albertus Geurkink
University of Groningen, Netherlands
Title: Transcriptomics as a tool to monitor and predict the behaviour of the microbiome in an industrial salt waste water treatment plant
Time : 17:45-18:00

Biography:
A K Geurkink works in the laboratory of G J W Euverink, at the Faculty of Science and Engineering, University of Groningen. He is also a part of Bioclear b.v, The Netherlands. His research interests are in Applied Microbiology, Biotechnology and Water Microbiology/bio-technology.
Abstract:
In waste water treatment plants, polluted water is purified with the help of complex ecosystems of many different bacteria. In these systems, many different desirable but also undesirable microbial activities play a role. To achieve detailed insight in the microbial activities of these processes in a waste water treatment plant, metagenomic tools can be applied. Until now, transcriptomics tools are almost exclusively reserved for applications in fundamental research. One of the reasons is that metagenomic techniques are complex and generates a very large dataset. Furthermore, in most cases it takes too long to obtain practical answers and solutions to questions raised in the ‘real world’. The costs associated with such an approach are also very high compared to the costs of measuring a set of standard physical and chemical parameters. However, transcriptomics results in an enormous amount of knowledge about the microbial processes in a waste water treatment plant that can be used to improve the quality of the effluent. Therefore, new pragmatic ways to bridge the gap between transcriptomics and business are required. This approach is characterized by the strong cooperation between fundamental research and a practical application. It distinguishes itself by combining Next Generation Sequence (NGS) data of DNA and RNA extracted from a waste water treatment plant at specific time points. NGS data will give detailed insight into the population and activities of a sample while (high throughput) quantitative-PCR is used to get insight into the dynamics of selected set of target genes. Mathematical analysis of the historical data combined with the chemical/physical characteristics of the influent water allows us to predict and control the microbial behavior and thereby the performance of the waste water treatment plant.
Claire Baranger
Université de Technologie de Compiègne, France
Title: “Myco-fluidics†for soil remediation: developing a microfluidic tool to monitor the solubilization and uptake of a persistant soil pollutant in filamentous fungi

Biography:
Claire Baranger graduated from the Ecole Supérieure de Biotechnologie de Strasbourg, France, in 2015. She is a trained biotechnologist with experience in microbiology and enzymology, and has a specific interest in mycology and environmental biotechnologies. She is currently pursuing a PhD at the Université de Technologie de Compiègne in France.
Abstract:
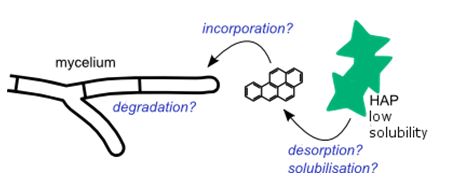
Bioremediation appears as a relatively low-cost solution for the restoration of soils contaminated with persistent organic pollutants. Among these pollutants, high molecular weight polycyclic aromatic hydrocarbons (PAHs) display a high resistance to degradation and low bioavailability.
Filamentous fungi are known for their ability to degrade complex substrates, and some soil fungi, such as Talaromyces helicus, showed promising results regarding the biodegradation of PAHs. However, the mechanisms involved in the incorporation and biodisponibility modifications of these pollutants remain poorly understood.
In this context, the Myco-fluidics project aims at using microfluidics to mimic the soil microenvironment and monitor the solubilization and uptake of pollutants in filamentous fungi in vivo. This approach allows direct microscopic observations at a cellular scale, in complement with a characterization of the degradation in macroscopic cultures.
Preliminary results show the intracellular storage of benzo[a]pyrene in T. helicus hyphae, and suggest the release of tensio-active compounds likely to promote the desorption and stabilization of HAPs in the aqueous phase. In a broader perspective, this soil-on-a-chip model could be used as a new tool to investigate parameters affecting the biodegradation of pollutants, in order to develop more efficient fungus-mediated soil cleanup strategies.
Recent Publications
- Bhardwaj, G. (2013). Biosurfactants from Fungi: A Review. J. Pet. Environ. Biotechnol. 4
- Fayeulle, A., Veignie, E., Slomianny, C., Dewailly, E., Munch, J.-C., and Rafin, C. (2014). Energy-dependent uptake of benzo[a]pyrene and its cytoskeleton-dependent intracellular transport by the telluric fungus Fusarium solani. Environ. Sci. Pollut. Res. 21, 3515–3523.
- Held, M., Edwards, C., and Nicolau, D.V. (2011). Probing the growth dynamics of Neurospora crassa with microfluidic structures. Fungal Biol. 115, 493–505.
- Marco-Urrea, E., García-Romera, I., and Aranda, E. (2015). Potential of non-ligninolytic fungi in bioremediation of chlorinated and polycyclic aromatic hydrocarbons. New Biotechnol. 32, 620–628.
- Stanley, C.E., Grossmann, G., Casadevall i Solvas, X., and deMello, A.J. (2016). Soil-on-a-Chip: microfluidic platforms for environmental organismal studies. Lab Chip 16, 228–241.
- Special Talk
Location: Redwood Suite C
Chair
TBD
TBD
Session Introduction
Alain Richard
National Research Council of Canada (NRC), Québec, Canada
Title: Tips for a Successful Technology Transfer in Life Sciences

Biography:
Alain Richard completed his BSc in Microbiology and MSc in Microbiology-Immunology & PhD). He has dedicated his 20 years’ career to innovation in biotechnology and biopharma, acting at crossroads between science and business: 8 years in biotech companies and more than 12 years in technology transfer in the Province of Québec, Canada (university or government - industry liaison). He is currently active in this particular field at the National Research Council of Canada as a Client Relationship Leader in Human Health Therapeutics.
Abstract:
The relevance of technology transfer has been demonstrated from the early stages of microbiology (e.g. industrial cases with Louis Pasteur). Technology transfer allows the deployment of science into society. It is an evolving field of knowledge combining science and business approaches. It is a process that requires focus and persistence. There are some essential parameters to consider in that process. There are intellectual property aspects such as confidentiality, patenting, individual and institutional commercial rights. The developmental stage of a technology is key to deciding what steps to take to increase the chances of success of the transfer. It means partnering with the appropriate organizations involved along the process. There are many mechanisms to add value to an invention disclosure to make it more amenable for a transfer in the forms of a license or a start-up.
Some typical agreements are commonly used in technology transfer, such as confidentiality agreements, material transfer agreements, R&D licenses with or without commercial option, commercial license agreements, and creation of spin-off companies. Excellent science does not necessarily mean an entry to the market. Understanding the drivers and the needs of a relevant market can prove to be difficult, but there are some ways to overcome this. There is a cultural encounter between business and science; both fields of knowledge have to reach common goals. The conditions sought in pharmaceutical sector for in-licensing are different than in other fields of life sciences due to the multi-million-dollar cost of clinical research trials prior to market authorization. There are common indicators used in technology transfers, from rules of thumb to complex financial valuations. This presentation aims to provide some insight on the elements described above.
- Industrial Microbiology, Food Microbiology, Vaccines and Anti-Microbials, Microbial Immunology, Molecular and Medical Microbiology
Location: Redwood Suite C

Chair
Sarah Louise Cosby
Queen’s University Belfast & Agri-Food & Biosciences Institute, UK

Co-Chair
Pietro Mastroeni
University of Cambridge United Kingdom
Session Introduction
Pavel Dibrov
University of Manitoba, Canada
Title: A novel antibiotic effectively inhibiting an unconventional target, Na+-translocating NADH:ubiquinone oxidoreductase
Time : 12:25-12:50

Biography:
Abstract:
Statement of the Problem: The present crisis in antibiotic development and administering was precipitated not only by decades of global misuse of broad-spectrum antibiotics and resulting proliferation of multi-drug resistant strains, but also by a general strategy in antibiotic design, when a very limited set of prokaryotic enzymes and metabolic pathways was ever targeted. The situation calls for urgent intensification of the search for non-traditional antimicrobial targets. Methodology & Theoretical Orientation: The Na+-translocating NADH:ubiquinone oxidoreductase (Na+-NQR) is a key respiratory enzyme in many aerobic pathogens, including a widespread and notoriously difficult to treat Chlamydia trachomatis. Inhibition of Na+-NQR was predicted to arrest bacterial energization and proliferation, and ultimately disrupt the infectious process. To examine this prediction, a new furanone inhibitor of Na+-NQR, PEG-2S, was designed and assayed for its anti-chlamydial activity in cell culture models of infection. Findings: The presented work confirmes that Na+-NQR is critical for the Chlamydia trachomatis infectious process. A newly designed PEG-2S inhibited intracellular proliferation of Chl. trachomatis with a half-minimal concentration in the submicromolar range without affecting the viability of mammalian cells or selected species representing benign intestinal microflora. Infection by Chl. trachomatis increased H+ and Na+ concentration in the infected mammalian cell. Addition of PEG-2S blocked these changes in ion balance induced by Chl. trachomatis infection. PEG-2S also inhibited the Na+-NQR activity in sub-bacterial membrane vesicles isolated from Vibrio cholerae when added at very low (nanomolar) concentrations. Conclusion & Significance: The obtained results demonstrate that Na+-NQR is critical for the bacterial infectious process and is susceptible to a precisely targeted bactericidal compound in situ. PEG-2S opens a new line of narrowly targeted inhibitors of NQR and is serving as a molecular platform for the development of “individually tailored” anti-NQR remedies narrowly targeting specific pathogens.
Boris V Nikonenko
Central Institute for Tuberculosis, Russia
Title: Activity of new indole derivatives against Mycobacterium tuberculosis and Mycobacterium avium in HIV combined mycobacteriosis
Time : 14:00-14:25

Biography:
Boris V Nikonenko MD, Ph.D., Doctor of Medical Sciences is a leading Researcher at Central Research Institute for Tuberculosis in Moscow. He is an expert in immunology and genetics of experimental tuberculosis and other mycobacterial infections, as well as testing of TB vaccines and drugs. Along with colleagues, he mapped three loci in the mouse genome that regulates the course of TB infection and immunity and revealed the role of other (known) genes in mouse ant-TB resistance and immunity. For 12 years, he worked in biopharm company Sequella, Inc. (Rockville, USA) testing the activity of established drugs against M. tuberculosis, M. avium, M. abscessus, Candida in the mouse models.
Abstract:
Given the data of high-level activities of original lead compounds, obtained earlier at All-Union Scientific Research Chemical-Pharmaceutical Institute (does not exist now) and A N Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences (INEOS RAS), we designed now about 300 multi-target compounds in which electron donor (indole and and ferrocene) and electron acceptor pyridine scaffolds, including INH, are linked via N-N-containing pharmacophore groups, either linear or within the heterocyclic structure. Compounds were tested at the Central Institute for Tuberculosis (CNIIT). Results demonstrated that about 40 agents exhibited proven high activity in vitro and ex vivo against both drug-sensitive M. tuberculosis Ð37Rv (MIC = 0.05-2 μg/mL) and the isoniazid (INH)-resistant clinical isolates of M. tuberculosis CN-40 (MIC = 0.018-4.44 μg/mL), as well as against M. avium (MIC = 0.05-1.5 μg/mL for 15 compounds) and other non-tuberculosis HIV co-infections. Thus, these agents were virtually as active as INH against M. tuberculosis H37Rv; however, unlike INH, those showed remarkable activity against the INH-resistant M. tuberculosis strain and M. avium. 3-triazeneindole TU112 demonstrated high level activity against dormant non-culturable M. tuberculosis. In addition, about 80 compounds among the tested ones show MIC values in the range of ≥2 and ≤ 10 μg/mL, thus ~40% of the compounds exhibited appreciable anti-TB activity. The compounds are hybrid molecules designed on a basis of privileged scaffolds – 3-indolyl (substituted at positions 1,2,5) and 2-4-pyridines including isoniazid one. The strategy of designing new agents based on a concept of molecular hybridization, as well as a recently emerging concept of development membrane-active (redox-mediated) agents as a strategy for treating persistent infections.
Recent Publications
1. Nikonenko B, Reddy VM, Bogatcheva E, Protopopova M, Einck L, Nacy CA (2014) Therapeutic efficacy of SQ641-NE against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 58(1): 587-9.
2. Kondratieva T, Azhikina T, Nikonenko B, Kaprelyants A, Apt A (2014) Latent tuberculosis infection: what we know about its genetic control? Tuberculosis (Edinb). 94(5): 462-8.
3. Velezheva V, Brennan P, Ivanov P, Kornienko A, Lyubimov S, Kazarian K, Nikonenko B, Majorov K, Apt A (2016) Synthesis and antituberculosis activity of indole-pyridine derived hydrazides, hydrazide-hydrazones, and thiosemicarbazones. Bioorg Med Chem Lett. 26(3): 978-85.
4. Moore JH, van Opstal E, Kolling GL, Shin JH, Bogatcheva E, Nikonenko B, Einck L, Phipps AJ, Guerrant RL, Protopopova M, Warren CA (2016) Treatment of Clostridium difficile infection using SQ641, a capuramycin analogue, increases post-treatment survival and improves clinical measures of disease in a murine model. J Antimicrob Chemother. 71(5): 1300-6.
5. Nikonenko BV, Kornienko A, Majorov K, Ivanov P, Kondratieva T, Korotetskaya M, Apt AS, Salina E, Velezheva V (2016) In vitro activity of 3-triazenoindoles against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 60(10):6422-4.
Pierre-Yves Morvan
Codif Technologie Naturelle, France
Title: Effects of stressful life on human skin biota

Biography:
Abstract:
Marcin Åukaszewicz
University of Wroclaw, Poland
Title: Microbial production of biopolymers using solid-state fermentation with Bacillus subtilis natto
Time : 16:45-17:10

Biography:
Marcin Åukaszewicz is a Polish biologist, with his research interest and specialization in the fields of molecular biology, biotechnology and microbiology. He did his PhD in Catholic University, Belgium and later became became an assistant professor at the Institute of Genetics and Microbiology, University of WrocÅ‚aw. He is currently the dean of the Department of Biotransformation, at the Faculty of Biotechnology, University of WrocÅ‚aw.
Abstract:
Biopolymers are obtained from a wide range of microorganisms and plants. Microbial polymers are produced by the fermentation process or by chemical polymerisation of monomers. They have a wide variety of applications and can potentially replace synthetic materials. Using industrial by-products to produce exopolysaccharides can reduce the costs associated with their biosynthesis and can solve the problem of waste management after their production. It is important to select an appropriate microorganism that does not endanger human health. Solid-state fermentation (SSF) has biotechnological advantages such as higher fermentation productivity and higher product concentration. Over past five years, there have been significant developments in this method, unfortunately these advances have mainly occurred at the laboratory scale. Bacillus subtilis natto is a well-known GRAS microorganism that produces the biopolymer levan. Levan is a polymer consisting of D-fructofuranose units joined by β-(2,6) linkages and is produced by both plants and bacteria. Microbial levan potentially has a very wide range of applications in the food industry, cosmetics, pharmaceuticals, and medicine. Industrial application of levan depends on its molecular weight, which can be modulated using different strains, as well as culture conditions such as temperature, pH, agitation, carbon source, and other medium components. The aim of this research was to obtain levan in SSF using rapeseed meal as the main substrate and different Bacillus subtilis natto strains. A new bioreactor for SSF was designed. Suitable methods for product separation and purification were also investigated. The purification process was performed with low-pressure liquid chromatography, using different types of column fillers. The molecular weight distribution of levan produced in the SSF process was determined using gel permeation chromatography, and 1H NMR (Proton nuclear magnetic resonance) was used to identify levan. We observed some differences in the molecular weight range of levan obtained from the cultivation of Bacillus strains in submerged fermentation and SSF. Various molecular weight levans may have different applications due to their specific properties.
Recent Publications
1. Esawy M A, Abdel-Fattah A M, Ali M M, Helmy W A, Salama B M, Taie H A A, Hasheman AM, Awad G E A (2013) Levansucrase optimization using solid state fermentation and levan biological activities studies. Carbohydrate Polymers 96: 332–341.
2. Kreyenschulte D, Krull R, Margaritis A (2012) Recent advances in microbial biopolymer production and purification. Critical Reviews in Biotechnology 34: 1–15.
3. Porras-Domínguez JR, Ávila-Fernández A, Miranda-Molina A, Rodríguez-Alegría ME, López Munguía A (2015) Bacillus subtilis 168 levansucrase (SacB) activity affects average levan molecular weight. Carbohydrate Polymers 132: 338–344.
4. Öner ET, Hernández L, Combie J (2016) Review of Levan polysaccharide: from a century of past experiences to future prospects. Biotechnology Advances 34: 827–844.
5. Thomas L, Larroche C, Pandey A (2013) Current developments in solid-state fermentation. Biochemical Engineering Journal 81: 146–161.
Petra Dersch
Helmholtz Centre for Infection Research, Germany
Title: Role of Type III secretion system and the CNFY toxin for the virulence of enteric Yersiniae

Biography:
Abstract:
Herbert B. Allen
Drexel University College of Medicine, USA
Title: Leprosy from a Different Perspective

Biography:
Herbert B Allen specialties include dermatology and dermatopathology, skin pathology and fungal infections. He is a graduate of Johns Hopkins University School of Medicine. He has served on the boards of the American Society of Dermatology and the American College of Physicians and has published over 30 scientific articles in the fields of dermatology and dermatopathology. He is the author of ‘Keywords’ in Dermatology, a book on the language of dermatology. He's board-certified with the American Board of Dermatology and the American Board of Pathology. He is currently an Emeritus Professor in the Department of Dermatology where he served as chair of the department for 14 years.
Abstract:
We have recently written about leprosy and its treatment that has proven to be an incredible achievement in medical history. This biblical disease has been brought under control and the worldwide incidence has decreased from 12,000,000 in 1975 to 800,000 in 2015. The only thing that changed in that time span was the addition of rifampin to the treatment regimen. Rifampin “pokes holes” in biofilms and allows dapsone to penetrate and kill the mycobacteria inside. Prior to rifampin, the organism was becoming resistant to dapsone. Clofazamine was also added, but it was useful mainly in relation to managing the reaction states in the disease, mostly erythema nodosum leprosum. Even though rifampin was added as another antibiotic because of resistance, its action was too short lived (3-5 hours half-life) for its activity given the prescribed dosage of once per month. Also, resistance develops rapidly with rifampin, so it seems evident it is behaving differently from its activity as an antibiotic. That different activity is biofilm dispersion. Biofilm is most assuredly present in leprosy because we have observed it not only in “globi” in the skin, but also in the liver, spleen, and kidneys as “secondary amyloidosis of chronic disease.” It stains histopathologically with PAS which stains the polysaccharides make up the bulk of the biomass and with Congo red that stains the amyloid that makes up biofilm infrastructure. TLR2 has been shown to be activated by biofilms, and TLR2 itself has been shown to activate IL4, IL 10 both of which are present in lepromatous leprosy. The fortuitous choice to include rifampin in the multidrug regimen reversed the course of leprosy and offers hope that other chronic diseases would behave similarly.
- Workshop
Location: Redwood Suite C
Session Introduction
Joachim Wink
Helmholtz Centre for Infection Research, Germany
Title: Polyphasic Taxonomy – The Class Actinobacteria

Biography:
Joachim Wink has completed his PhD in 1985 from Frankfurt University. He then went to the pharmaceutical industry and started his career at the Hoechst AG, where he was responsible for the strain collection and specialized in the cultivation and taxonomic characterization of Actinobacteria and Myxobacteria. During the years, he was responsible for the strain library within the pharmaceutical research and a number of screening projects with Hoechst Marion Russel, Aventis and Sanofi. In the year 2005, he did his habilitation at the Carolo Wilhelma University of Braunschweig and in 2012, he went to the Helmholtz Centre for Infection Research in Braunschweig, where he founded the working group of the strain collection with its focus on Myxobacteria. Here, he is now working on the isolation and taxonomic characterization of Myxobacteria and Actinobacteria, as well as, the analysis of their secondary metabolites. He has published more than 50 papers on secondary metabolites and the taxonomy of the producing microorganisms in reputed journals, a number of reviews, as well as, book chapters and more than 35 patents. He is the Editorial Board Member of a number of international journals.
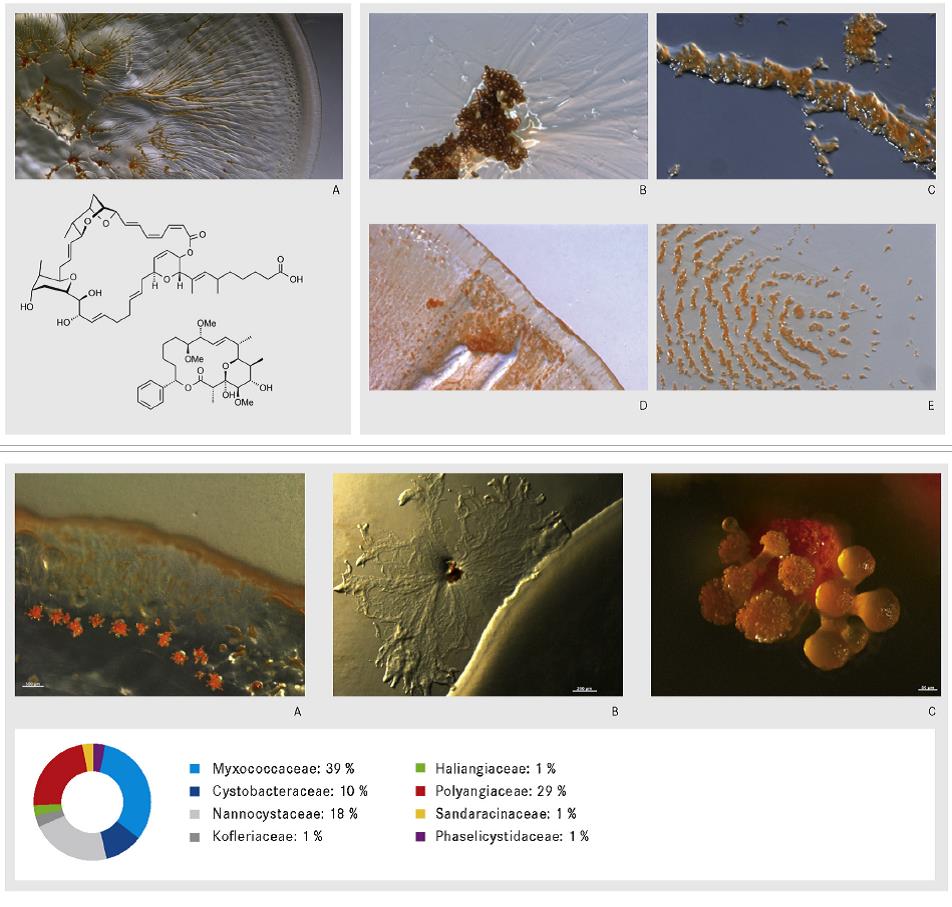
Abstract:
The myxobacteria with the order Myxococcales (TCHAN, POCHON and PRÉVOT 1948) belong to the Gram negative Proteobacteria and have first been described in detail by Thaxter in 1892 in the Botanical Gazette. Most known myxobacteria occur in soil and frequently develop on decomposing plant material, the bark of living trees or animal dung. Both in nature and in the laboratory their presence may be detected through the appearance of fruiting bodies. Since the introduction of Epotilon as anticancer therapeutic on the market, the myxobacteria have their place in the group of industrial important bacteria. The biology of myxobacteria is mainly characterized by two features that are gliding on surfaces without any locomotion organelles and the formation of fruiting bodies. Myxobacteria also have a high GC contend and very huge genomes with a size of about 10 Mb which correlates with their ability to produce many different secondary metabolites. Until today, the taxonomy of the myxobacteria is basing on the morphological features together with the 16S rRNA sequence, in addition, we have established a number of chemotaxonomic and molecular biological markers, which can be used in myxobacterial taxonomy. A short overview on the history of myxobacteria, the fruiting body formation, its taxonomic classification and additional methods for characterization, as well as, their potential to produce bioactive compounds is given in this talk.