Prof. Martin Ryan
University of St Andrews, UK
Title: Protein biogenesis in the Picornaviridae : Virus-encoded proteinases, ribosome ‘skipping’, alternative initiation and programmed ribosomal frame-shifting
Biography
Biography: Prof. Martin Ryan
Abstract
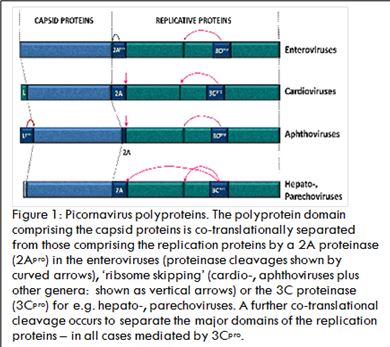
The genome structure of viruses within the Picornavirus family is similar to cellular mRNAs: +v sense RNA genomes comprising a long 5’ non-coding region (NCR), a single open reading frame (ORF; ~2,300 aa), a short 3’-NCR with a poly(A) tail, although the 5’ cap structure is quite different. When introduced into the cytoplasm, the virus RNA can function directly as a messenger RNA. The challenge faced by these viruses is, however, the need to generate a multiplicity of different proteins (capsid, RNA replication proteins) from this single ORF. All picornaviruses encode a proteinase (3Cpro) which ‘processes’ the polyprotein into mature products by a combination of a single, co-translational, intramolecular cleavage (in cis) and multiple post-translational intermolecular cleavages (in trans). Some picornaviruses have acquired a second proteinase (aphthoviruses – Lpro; enteroviruses – 2Apro) which perform a single co-translational cleavage in cis, but then go onto cleave cellular proteins involved in the cap-dependent translation of host-cell mRNAs and proteins involved in interferon production in response to infection. The 2A region of aphtho- and many other Picornavirus genera (unlike enteroviruses not a proteinase) may either be a short oligopeptide sequence, or, a larger protein that mediates a translational ‘recoding event termed ‘ribosome skipping’ at the C-terminus of 2A such that translation stops (releasing the nascent protein) then recommences translation of the down-stream replication protein sequences: an apparent ‘cleavage’, but in actuality a discontinuity in the polypeptide backbone. It is now known that this form of control over protein biogenesis is used by other types of RNA virus and some cellular genes. Uniquely within the picornaviruses, Theiler's murine encephalomyelitis virus (TMEV; genus Cardiovirus) encodes an overlapping, alternative ORF within the leader, or L protein, alternative initiation producing the L* protein which plays an important role in the establishment of persistent CNS infections by TMEV. Recently it has been shown that some picornaviruses also use programmed ribosomal frame-shifting shortly after the ribosome skipping event to (translationally) down-regulate the production of replication proteins at later stages of the infectious cycle. Furthermore, this frame-shifting does not rely solely on an RNA secondary structure but is directed by protein 2A. In conclusion, the picornaviruses have evolved a range of different mechanisms to control their protein biogenesis: not at the level of RNA transcription, but by co- and post-translational events alone.
Recent Publications
- Ryan MD, Flint M (1997) Virus-encoded proteinases of the picornavirus super-group. J. Gen. Virol. 78: 699-723.
- Donnelly MLL, Luke G, Mehrotra A, Li X, Hughes LE, Gani D, Ryan MD (2001) Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J. Gen. Virol. 82:1013-1025.
- Sharma P, Yan F, Doronina V, Escuin-Ordinas H, Ryan MD, Brown J (2012) 2A peptides provide distinct solutions to driving stop-carry on translational recoding. Nuc. Acids Res. 40:1-9.
- Chen HH, Kong WP, Zhang L, Ward PL, Roos RP (1995) A picornaviral protein synthesized out of frame with the polyprotein plays a key role in a virus-induced immune-mediated demyelinating disease. Nat. Med. 1:927-931.
- Napthine S, Ling R, Finch LK, Jones JD, Bell S, Brierley I, Firth AE (2017) Protein-directed ribosomal frameshifting temporally regulates gene expression. Nat. Commun. 8:15582.